Table of Contents
MVS Pharma GmbH Quality System Overview: At MVS Pharma GmbH, quality is not merely a compliance requirement; it is the foundation of our identity and operations. Following our successful EU GDP inspection in 2024 by the Stuttgart Authority, MVS Pharma proudly demonstrates that our Quality Management System (QMS) is both robust and forward-looking. It ensures the integrity of every product we handle, from biotechnology innovations to drug-device combination products.
Building a Quality-Driven Organization
MVS Pharma’s Quality Policy defines the orientation of our company; uniting mission, vision, and values into a cohesive framework that guides every action.
Our mission drives innovation in biotechnology and pharmaceuticals, focusing on antiviral medical devices and essential supplements to ensure healthier lives and reliable access to treatments. Our vision, rooted in the principle of “State of Science and Technology,” commits us to continuous improvement, ensuring our products and services reflect the latest scientific standards.
This philosophy shapes our QMS, aligning fully with the EU GDP Guidelines (2013/C 343/01). Every process, from procurement to distribution, is governed by documented procedures and real-time oversight, ensuring traceability, reliability, and patient safety.
MVS Quality Management System: Structure and Approach
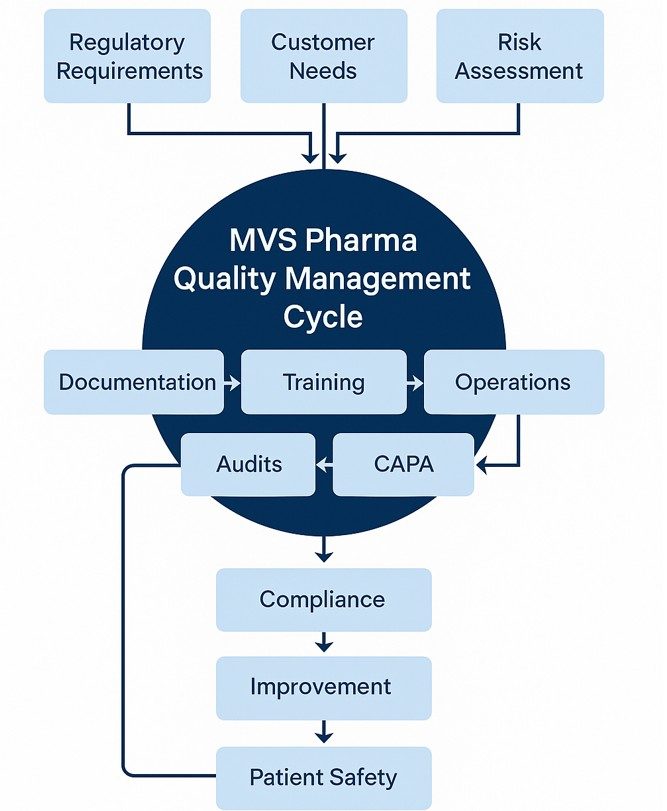
MVS Pharma GmbH Quality System is built on a structured yet agile framework that connects regulatory compliance with operational excellence. The system encompasses all areas of GDP, including management responsibility, training, documentation, operations, deviation control, risk management, and continuous improvement.
- Digital QMS Infrastructure:
MVS utilizes a digitalized Quality Management System, leveraging the Microsoft Office Professional Suite as an integrated platform for controlled documentation, CAPA tracking, change control, and training management. This setup ensures data integrity, version control, and efficient communication across departments.
- Risk-Based Thinking:
Every quality-related activity – from supplier qualification to temperature monitoring- is guided by proactive risk assessments. This ensures that potential deviations are identified and mitigated before they affect product quality or patient safety.
- Competence and Training:
Employee competence is a key pillar of our quality culture. Under SOP HR-SOP-02-EN_Training, all personnel receive GDP-focused training that enhances awareness and consistency in operational practices. Training effectiveness is reviewed during the Management Review Meeting (MRM) to promote continual improvement.
- Process Oversight and Continuous Improvement:
MVS maintains a structured internal audit and deviation management process to ensure compliance and identify opportunities for improvement. Lessons learned are systematically integrated into revised SOPs and work instructions.
- Culture of Integrity:
Our quality values – compliance, transparency, respect, and patient-first decision-making- create a strong ethical foundation. Every employee is encouraged to act as a “guardian of quality,” ensuring the principles of the EU GDP are not just implemented, but lived daily.
Key Strengths of the MVS Pharma GmbH Quality System
- Regulatory Alignment: 100% compliance with EU GDP, AM-HandelsV, and AMWHV national regulations.
- Digital Efficiency: Controlled document management and process visibility through the Microsoft Office QMS framework.
- Training Excellence: Structured, recurring, and effectiveness-evaluated training cycles.
- Traceability & Documentation: End-to-end traceability across all operations and partners.
- Risk & CAPA Management: Integrated assessment, root cause analysis, and preventive controls.
- Audit Readiness: Regular self-inspections and management reviews ensure readiness for authority inspections at any time.
Conclusion
MVS Pharma GmbH Quality System: The success of MVS Pharma GmbH’s EU GDP inspection stands as a testament to our commitment to quality, compliance, and continuous improvement. Our Quality Management System is not just a regulatory obligation – it is the living backbone of a company dedicated to protecting patients, empowering employees, and driving pharmaceutical excellence through science, technology, and integrity.
FAQs
-
What does EU GDP certification mean for a pharmaceutical company like MVS Pharma?
EU GDP (Good Distribution Practice) certification confirms that a company meets all European standards for the proper distribution, storage, and handling of medicines, ensuring product quality and patient safety throughout the supply chain.
-
How did MVS Pharma achieve full compliance with EU GDP requirements?
MVS Pharma established a fully digital Quality Management System (QMS) aligned with all EU GDP guidelines, integrating risk-based processes, staff training, and full traceability from sourcing to distribution.
-
What makes MVS Pharma’s Quality Management System different from traditional systems?
Unlike paper-based systems, MVS Pharma’s QMS is fully digitalized through Microsoft Office Professional Suite, allowing real-time documentation control, CAPA tracking, and streamlined communication across departments.
-
How does MVS Pharma ensure data integrity and traceability?
All quality data is securely version-controlled, with every product movement traceable across partners and processes, ensuring full compliance with GDP and AMWHV regulations.
-
How often are employees trained on GDP and quality procedures?
GDP-focused training sessions are held regularly under our HR-SOP-02-EN_Training program. Each cycle’s effectiveness is evaluated during Management Review Meetings to ensure continuous improvement.
-
What role does risk management play in MVS Pharma’s operations?
Risk assessments are integrated into every quality-related activity—anticipating potential deviations before they occur and safeguarding both product quality and patient safety.
-
How does MVS Pharma stay audit-ready at all times?
Through regular internal audits, self-inspections, and management reviews, MVS maintains continuous compliance and readiness for unannounced inspections by health authorities.
-
What values guide MVS Pharma’s approach to quality and compliance?
MVS Pharma’s culture is founded on compliance, transparency, integrity, and patient-first decision-making—empowering every employee to act as a guardian of quality.


