Table of Contents
EU GDP quarantine and inspection: Quarantine and inspection are essential control points within the EU Good Distribution Practice (GDP) framework. They ensure that medicinal products are not made available for distribution until their identity, integrity, and compliance have been verified. At MVS Pharma GmbH, quarantine and inspection processes are designed to prevent unauthorized use, eliminate risks associated with damaged or nonconforming products, and ensure that only approved products enter controlled storage and subsequent distribution.
EU GDP Expectations for Quarantine & Inspection
EU GDP requires that all medicinal products received are quarantined until their acceptability is confirmed. During this period, products must be clearly identified, physically or systemically segregated, and protected from accidental or unauthorized use. Inspection activities must verify that products meet defined requirements before release, and all decisions must be documented and traceable.
Key GDP principles include:
- clear segregation of quarantined goods
- controlled access to quarantined stock
- documented inspection and release decisions
- Responsible Person (RP) oversight
- defined handling of nonconforming goods
MVS Pharma’s Quarantine and Segregation Controls
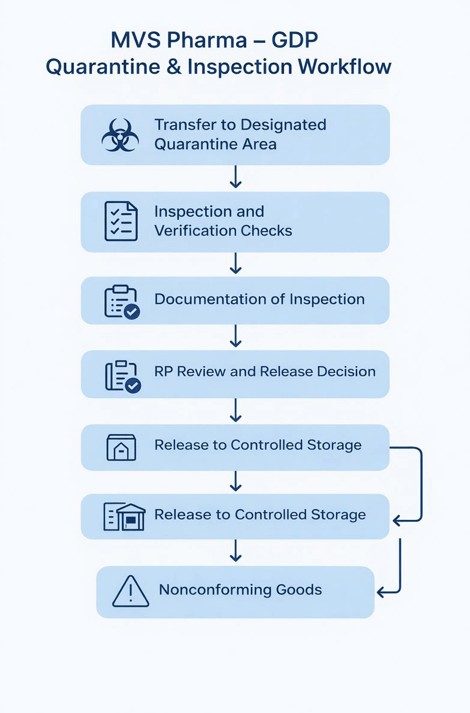
- Designated Quarantine Area
At MVS Pharma GmbH, all incoming medicinal products are transferred directly from goods receipt to a clearly designated Quarantine Area. This area is physically segregated from approved and rejected stock and is accessible only to authorized personnel.
Products under quarantine are visually identified using appropriate status labeling, ensuring immediate recognition of their non-released status and preventing unintended distribution.
- Quarantine Status Management
Quarantine status is controlled through a combination of physical identification and documented records. Products remain under quarantine until inspection activities are completed and a formal release decision is taken. No product is permitted to move into approved storage without documented authorization.
This dual control approach ensures robust traceability and full compliance with GDP expectations.
Inspection Activities at MVS Pharma – EU GDP Quarantine and Inspection
- Comprehensive Inspection Checks
During the inspection phase, trained personnel verify the following:
- product identity and description
- batch number and expiry date
- packaging integrity and absence of tampering
- correctness and legibility of labeling
- results of temperature-at-receipt checks
- transport condition compliance
- completeness and accuracy of accompanying documentation
- Inspection activities are performed using structured checklists to ensure consistency and completeness.
- Documentation of Inspection and Release
Inspection results are documented in controlled records, including inspection reports and release documentation. These records provide full traceability of the inspection outcome and support regulatory review during audits or inspections.
All records are maintained within MVS Pharma’s Digital Quality Management System in accordance with ALCOA+ data integrity principles.
Decision-Making and Release Authority
- Responsible Person Oversight
While trained warehouse or quality personnel conduct the inspection, the final decision to release products from quarantine is made exclusively by the Responsible Person (RP). This ensures independent quality oversight and regulatory accountability.
Once approved, products are formally released and transferred to the temperature-controlled storage area under defined conditions.
Handling of Nonconforming or Suspect Goods
- Controlled Management of Deviations
If any discrepancies, damage, or deviations are identified during inspection, products remain under quarantine or are transferred to a designated Nonconforming / Blocked Area. A deviation or nonconformance report is initiated, and a documented investigation is performed.
The RP evaluates findings and determines the appropriate disposition, which may include rejection, return to the supplier, or further investigation.
Traceability and Record Retention
- Full Traceability and Control
Quarantine duration, inspection outcomes, and release decisions are fully traceable through documented records. No product is released without documented approval, and all inspection and quarantine records are archived in accordance with defined retention requirements.
Trending of inspection findings and supplier-related discrepancies supports continuous improvement and risk management.
Conclusion
MVS Pharma GmbH remains committed to maintaining a robust, transparent, and EU GDP–compliant quarantine and inspection process. Through clear segregation, structured inspections, comprehensive documentation, and strong Responsible Person oversight, MVS ensures that only compliant, verified, and safe medicinal products are released for storage and distribution.


