Table of Contents
MVS Pharma GDP Compliant Goods Receipt Process: The Goods Receipt process is the first critical step in the pharmaceutical distribution chain and plays a decisive role in ensuring the quality, safety, and authenticity of medicinal products. Under EU GDP requirements, every batch entering the warehouse must be checked, documented, and verified with diligence before it can be released into the storage environment. At MVS Pharma GmbH, the Goods Receipt process is designed to prevent risks before they enter the system, ensuring complete assurance that only compliant, correctly handled, and fully traceable products enter the supply chain.
EU GDP Requirements for Goods Receipt – MVS Pharma GDP Compliant Goods Receipt Process
EU GDP Chapter 5 outlines strict requirements to ensure that products received are:
- obtained only from authorized suppliers
- delivered under appropriate transport and temperature conditions
- free from damage, tampering, or falsification
- fully traceable through documented checks
- examined by competent personnel before acceptance
Every step must be recorded, legible, accurate, and retrievable, ensuring transparency and accountability during inspections.
MVS Pharma’s GDP-Compliant Goods Receipt Process
- Verification of Supplier Authorization
Before goods are accepted, MVS Pharma verifies the supplier’s regulatory status in the EudraGMDP database. This ensures that products originate only from licensed and compliant wholesale distributors or manufacturers. This step directly supports falsified-medicine prevention and ensures full regulatory assurance.
- Visual and Physical Inspection of Delivered Goods
Upon arrival, trained warehouse staff perform a detailed examination of the shipment. This includes:
- inspection of the delivery vehicle’s general condition
- assessment of cleanliness, odors, leaks, or contamination risks
- verification that transport conditions were maintained (where applicable)
- inspection of outer packaging for dents, tears, punctures, moisture, or tampering
- verification of batch numbers, product names, quantities, and expiry dates
Any sign of damage or discrepancy results in immediate documentation and escalation to the RP.
- Temperature Check at Receipt
To ensure that medicinal products have been transported within specified limits, temperature is checked at the point of receipt using GDP-compliant thermometers.
All readings are documented in the Temperature-at-Receipt Log.
If a temperature deviation is detected:
- A Deviation Report is initiated
- The RP reviews the potential impact on product quality
- Goods remain under quarantine until a decision is made
This ensures full protection of temperature-sensitive products before they enter storage.
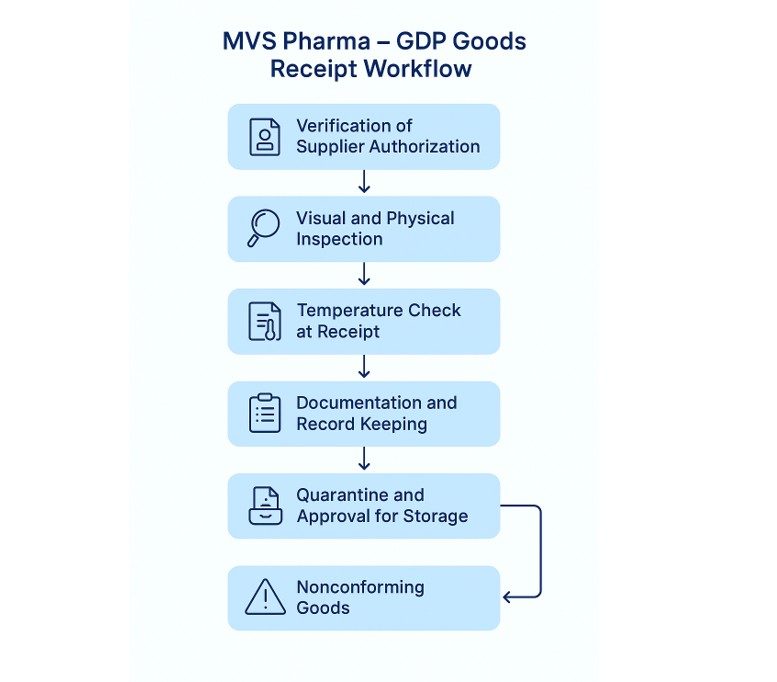
- Documentation and Record Keeping
The following documents are created as part of the Goods Receipt process:
- Receipt Ticket
- Receipt and Inspection Report
- Temperature-at-Receipt Log
- Damage / Deviation Report (if applicable)
All records are completed in real time, reviewed for accuracy, and stored under MVS Pharma’s Digital Quality Management System (Microsoft Office Suite).
This ensures compliance with ALCOA+ data integrity principles.
- Quarantine and Approval for Storage
After the initial checks are complete, all products are moved directly to the designated Quarantine Area.
This prevents premature access and ensures segregation from approved stock.
Only after the RP reviews the following can goods be “Released to Stock” and transferred into the controlled warehouse environment (15–25°C, RH < 60%).:
- supplier authorization
- inspection findings
- temperature compliance
- deviation reports (if any)
- Handling of Nonconforming Goods
If goods fail any part of the Goods Receipt process, MVS follows a controlled procedure that may include:
- segregation in a “Nonconforming Material Area.”
- documented investigation
- communication with the supplier
- RP decision regarding acceptance, rejection, or further evaluation
This ensures clear accountability and traceability of all rejected or potentially compromised products.
Conclusion
MVS Pharma GmbH is committed to ensuring that every product entering its facility undergoes a rigorous, transparent, and EU GDP-compliant Goods Receipt process. By combining trained personnel, structured documentation, and strong quality oversight, MVS guarantees that only safe, authentic, and fully compliant medicinal products enter its supply chain and ultimately reach patients.


