Importation of Medicinal Products Germany: In today’s global pharmaceutical landscape, the importation of medicinal products from non-EU/EEA (European Union/European Economic Area) countries is increasingly common. Since manufacturers and distributors seek to leverage diverse manufacturing capabilities and cost efficiencies. However, the process of importing medicines into the EU is highly regulated. The reason is to ensure product quality, safety, and efficacy for patients.
Table of Contents
Importation is not merely a logistical matter but a complex regulatory process involving detailed quality assurance and compliance obligations. On August 21, 2022, the new GMP Annex 21 comes into effect. It outlines the GMP requirements applicable to Import Authorization (IA) holders when importing medicinal products from outside the EU/EEA.
As MVS Pharma’s medicinal product suppliers are located outside the EU/EEA, we are also involved in the preparation and approval process of Import Authorization. In this article, you will learn about the essential regulatory requirements and considerations in importing medicinal products from non-EU/EEA countries.
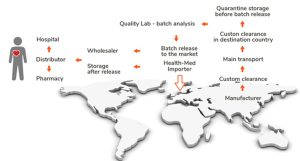
Understanding Import Authorization in the EU
An Import Authorization (IA) is a critical requirement for any company intending to bring medicinal products from non-EU/EEA countries into the EU market. Only companies holding this authorization, known as Import Authorization Holders (IAHs), can legally import these products.
This is compulsory for IAH to ensure the necessary infrastructure, processes, and quality management system (QMS) in place to handle, test, and store imported products in compliance with Good Manufacturing Practices (GMP).
Role of the Qualified Person (QP)
One of the central elements in the importation process is the role of the Qualified Person (QP). The QP is a highly trained and certified individual responsible for ensuring that each batch of imported medicine meets EU standards before it is released for sale or distribution.
MVS Pharma has highly qualified and trained QPs to review and verify the manufacturing documentation for each batch. This way, we ensure that testing, particularly for identity, strength, and purity, has been conducted per EU requirements. Each batch is thus certified before it enters the EU market.
Good Manufacturing Practice (GMP) Compliance (Importation of Medicinal Products in Germany)
For medicinal products manufactured outside the EU/EEA, demonstrating GMP compliance is a prerequisite for importation. If the manufacturing site in a non-EU/EEA country has been inspected and certified by an EU authority, the importer can rely on this certification. If not, it falls to the QP of the IAH to verify that the product was manufactured according to EU GMP standards.
Quality Control and Batch Testing
Before an imported product can be distributed within the EU, each batch must undergo quality control testing.
Where there is no mutual recognition agreement (MRA), it is a legal requirement to test each batch upon import into the EU. This must be done prior to certification by a competent inspection body. After that, the assessment is released in accordance with Annex 16 of the GMP Guide.
MVS Pharma outsources batch testing from an EU GMP-certified Italian lab to verify the results of the following tests:
- Identity Testing: Ensures that the product matches its label description.
- Potency Testing: Verifies the strength and efficacy of the active ingredients.
- Purity Testing: Confirms the product is free from impurities that could affect its safety or efficacy.
Customs Clearance and Documentation
Medicinal products from non-EU/EEA countries must undergo customs clearance, where documentation accuracy is paramount. Errors can lead to costly delays or shipment rejection. Importers must submit critical documents, including a Certificate of Analysis (CoA), GMP documentation, shipping records, and import authorization.
Stability and Storage Requirements (Importation of Medicinal Products in Germany)
Medicinal products imported into the EU must be stored according to strict stability and storage requirements to preserve product quality. Importers must have facilities equipped to maintain the required temperature, humidity, and environmental conditions.
MVS Pharma has a temperature-mapped warehouse with an automatic temperature monitoring system. This space is used for storing medicinal products as per labeled storage conditions.
Conclusion (Importation of Medicinal Products in Germany)
The importation of medicinal products from non-EU/EEA countries is a highly regulated process aimed at safeguarding patient health by ensuring product quality, safety, and efficacy. Importers must navigate a complex regulatory landscape that includes GMP compliance, quality control, and batch testing requirements.
Disclaimer:
As a service to our readers, MVS Pharma GmbH publishing provides access to our archived content library in our blog. Please note the date of the last review or update on all articles. No content on this site should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.
MVS Pharma GmbH will soon be launching an omega-3 dietary supplement onto the European market that has been developed for the highest quality standards in terms of oxidation avoidance and, therefore, greatest bioavailability. In addition, in vitro studies are currently underway at the University of Ulm, in which Professor Dr. Rüdiger Groß tested a patented mouth and nose spray (Virudol) that can eliminate various flu viruses based on natural substances.
In addition, MVS has a wholesale license and has specialized in sourcing much-needed medicines such as Amoxicillin, Salbutamol, etc. from India through its local branch with a focus on local quality and safety testing, compliance with international GMP regulations and the highest quality level of user security (examples of local language brochures, identical units of measurement, batch control and full tracking, etc.).


