Table of Contents
What is the QMS Pyramid? The QMS documentation structure is a hierarchical organization of documents within the QMS. The documentation hierarchy makes it easy to understand, communicate, and visualize the documentation structure. Each documentation level in the structure builds upon the previous one and contributes to the overall effectiveness of the QMS.
In the pharmaceutical industry, a robust Quality Management System (QMS) is essential for ensuring product safety, efficacy, and compliance with regulatory standards. The QMS pyramid organizes documents into four levels, each with a distinct role in upholding quality standards. This article will explain each level of the QMS pyramid and its role in maintaining a compliant and effective Pharmaceutical Quality System (PQS).
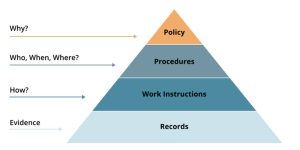
Level 1: Quality Policy/Manual
The Quality Manual sits at the top of the QMS pyramid and provides an overarching framework for the entire Quality Management System. It outlines the pharmaceutical company’s commitment to quality, defining policies, quality objectives, and the scope of the QMS. In a pharmaceutical setting, the Quality Manual often includes references to Good Manufacturing Practices (GMP) and relevant regulatory standards, such as those set by the European Medicines Agency (EMA) or the U.S. Food and Drug Administration (FDA).
Content typically includes:
- The scope and objectives of the QMS
- Quality policies aligned with regulatory requirements
- Roles and responsibilities within the QMS
- Cross-references to applicable standards and regulations (e.g., EU GMP, EU GDP, AMG, etc)
The Quality Manual sets the tone and expectations for all other documents within the QMS, creating a foundation for compliance and consistency across all operations.
Level 2: Procedures (QMS Pyramid)
Procedures represent the second level of the QMS pyramid and provide the “Who, When, Where” framework for critical processes within the organization. Pharmaceutical procedures cover a broad range of operations, including production workflows, document control, supplier qualification, training, and the management of non-conformances. They clearly define who is responsible for specific activities, the steps required, and any necessary approvals.
Level 3: Work Instructions
Work Instructions form the third level of the QMS pyramid. These documents provide “how-to”, detailed, task-specific guidance that builds on the general instructions outlined in Level 2 procedures. In the pharmaceutical industry, where accuracy and consistency are paramount, WIs serve as critical tools for training and daily operations.
Content typically includes:
- Step-by-step instructions for executing tasks
- Equipment settings and calibration requirements
- Diagrams, flowcharts, or visual aids (as needed)
- Safety considerations and handling instructions
These documents help reduce human error, ensure consistency, and are critical for new employee training, especially in production and quality control areas.
Level 4: Records (QMS Pyramid)
The Records at the base of the QMS pyramid serve as evidence of compliance and proof that the processes are executed as prescribed. These documents act as records of activities and operations, capturing essential data for tracking, traceability, and auditing purposes.
In the pharmaceutical industry, maintaining accurate records is not only a best practice but a regulatory requirement. Records and forms provide the documentation needed for internal and external audits, regulatory submissions, and product release decisions. Examples include completed forms, batch records, validation reports, training logs, and inspection records.
Conclusion
In essence, the QMS pyramid in the pharmaceutical industry structures documents to ensure clarity, consistency, and compliance across all operations. From the high-level Quality Manual, which sets the organization’s quality framework, down to the records that provide evidence of compliance, each level of documentation plays a vital role in maintaining a rigorous Pharmaceutical Quality System.
Disclaimer:
As a service to our readers, MVS Pharma GmbH publishing provides access to our library of archived content in our blog. Please note the date of the last review or update on all articles. No content on this site should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.
MVS Pharma GmbH will soon be launching an omega-3 dietary supplement onto the European market that has been developed for the highest quality standards in terms of oxidation avoidance and, therefore greatest bioavailability. In addition, in vitro studies are currently underway at the University of Ulm, in which Professor Dr. Rüdiger Groß tested a patented mouth and nose spray (Virudol) that can eliminate various flu viruses based on natural substances.
In addition, MVS has a wholesale license and has specialized in sourcing much-needed medicines such as Amoxicillin, Salbutamol, etc. from India through its local branch with a focus on local quality and safety testing, compliance with international GMP regulations and the highest quality level of user security (examples of local language brochures, identical units of measurement, batch control and full tracking, etc.).


